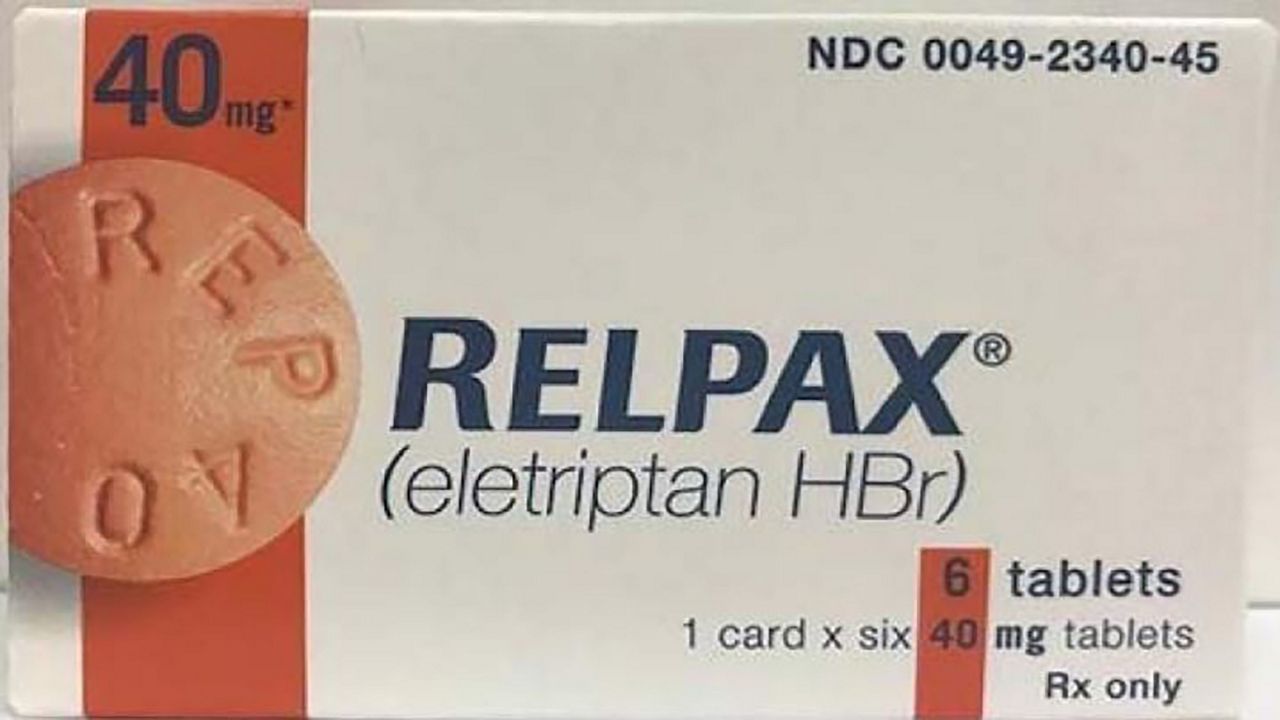
WASHINGTON, DC – Migraine sufferers check your medication. Pfizer Inc. is voluntary recalling RELPAX.
The U.S. Food and Drug Administration announced the company is recalling some of the medication because it failed to meet internal standards and could contain bacteria which could lead to illness.
The affected lots of 40mg tablets were distributed nationwide to wholesalers, retailers, hospitals and healthcare providers in the United States and Puerto Rico from June 2019 to July 2019. The two lots affected are:
- AR5407
- CD4565
If consumers ingest the potentially contaminated pills there is a risk the bacteria could enter their bloodstream and lead to serious, life-threatening infections.
If you have any of the drugs being recalled, stop using it immediately. Let your doctor know and return the drugs to your pharmacy. You can also contact Stericycle Inc., a company that specializes in medical waste removal, at 1-800-332-1088.